Page 2 of 3
Posted: Thu Dec 24, 2009 7:38 pm
by RYANSCHLESINGER
I already said it would be highly unlikly that it liquifies.
Posted: Thu Dec 24, 2009 7:42 pm
by D_Hall
Heimo wrote:all I am saying is I am fairly certain that the air would go liquid at the pressure
Incorrect. Air is beyond the critical temperature. There is no such thing as air liquification in the absense of cryogenic cooling.
That said, our poster is deluding himself if he thinks he's building the world's most powerful pneumatic cannon on anything that resembles a hobby budget. I don't know ALL the specs, but at the Naval Air Warfare Center - China Lake there exists a gun... It used to be called the "MIKES" gun(*) but they recently renamed it. In any event, the rough specs are something like....
6" bore x 30' barrel.
15 cubic foot x 3500 psi chamber using either dry nitrogen or helium depending upon exact shot requirements.
Note that I don't believe that the MIKES gun is the largest. I've no doubt that there are larger, more powerful guns elsewhere. I merely point it out as "a powerful pneumatic gun that no normal hobbyist has the budget to compete with."
Oh, and he's also wrong in that 10,000 psi air doesn't expand any faster than 3,000 psi air. It expands further, no doubt there. But not any faster.
(*) It was officially known as the "MIssile Kinematic Engagement Simulator", but the truth is it was invented by a guy by the name of Mike Gray. Mike's gun. Get it?

Posted: Thu Dec 24, 2009 7:52 pm
by rp181
Look up partial pressures.
Frankly,you sound like you don't know what your talking about. If your going for the largest pneumatic, why would you use air? helium would be the smart choice, and you can buy it in high pressure quantities.
At this point, unless you have a specific application, adding more pressure is just pointless, I would throw some thermal energy in there too.
Posted: Thu Dec 24, 2009 8:04 pm
by Heimo
D_Hall wrote:Heimo wrote:all I am saying is I am fairly certain that the air would go liquid at the pressure
Incorrect. Air is beyond the critical temperature. There is no such thing as air liquification in the absense of cryogenic cooling.
Ok I forgot for a moment that as the pressure rises so will the temperature I guess forgetting stuff like that comes easy at 3 am when I really should be asleep
Posted: Thu Dec 24, 2009 8:08 pm
by D_Hall
Heimo wrote:Ok I forgot for a moment that as the pressure rises so will the temperature I guess forgetting stuff like that comes easy at 3 am when I really should be asleep
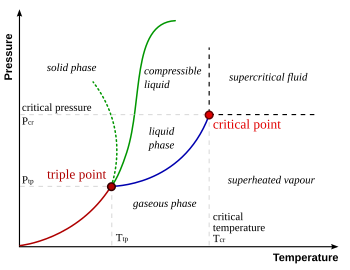
No, you really don't get it. Even at room temperature, nitrogen and oxygen (read: air) are both insanely hot (by their standards)! THEY WILL NOT LIQUIFY IN THE ABSENCE OF CRYOGENIC TEMPERATURES. Even room temperature is too damned hot! Take room temperature nitrogen and compress it to 1,000,000 psi. Know what you've got? A really dense gas refered to as a "supercritical fluid" but make no mistake... A "supercritical fluid" is NOT a liquid!
Look and learn. Pay particular attention to the "critical point" and the region above and to the right of it.
Posted: Thu Dec 24, 2009 8:10 pm
by Moonbogg
Heimo wrote:D_Hall wrote:Heimo wrote:all I am saying is I am fairly certain that the air would go liquid at the pressure
Incorrect. Air is beyond the critical temperature. There is no such thing as air liquification in the absense of cryogenic cooling.
Ok I forgot for a moment that as the pressure rises so will the temperature I guess forgetting stuff like that comes easy at 3 am when I really should be asleep
I'd say I forgot to, but the fact is I had no friggin idea in the first place!
Posted: Thu Dec 24, 2009 8:29 pm
by rp181
on the other end, helium will not solidify no matter how low the temperature is unless there is pressure.
http://en.wikipedia.org/wiki/Critical_p ... odynamics)
http://en.wikipedia.org/wiki/Supercritical_fluid
Posted: Thu Dec 24, 2009 9:19 pm
by kenbo0422
So, a supercritical fluid is like a gas at saturation??... ready to go liquid?? Or possibly hovering between the two stages??

Posted: Thu Dec 24, 2009 10:09 pm
by D_Hall
kenbo0422 wrote:So, a supercritical fluid is like a gas at saturation??... ready to go liquid?? Or possibly hovering between the two stages??

A thought experiment for you....
Put some water in a pressure vessel and purge it so that there's nothing but water and water vapor in said vessel. Seal the vessel.
Now start heating it up. What happens? Things get hotter, of course. But what else happens? Several things.
- The water increases in volume (as all things do when they get hotter). IE, THE WATER BECOMES LESS DENSE.
- The amount of water in the vapor phase (ie, steam) increases.
- The steam pressurizes due to temperature, yes, but it is significant to note that it also increases in pressure due to more mass (remember the increased amount of water in the vapor phase?) and the smaller volume it has to fill (remember that the water expanded?). IE, THE STEAM BECOMES MORE DENSE.
Continue heating the water. What happens? More of the same, of course. But the significant parts for my discussion is that the water continues to DECREASE in density while the steam continues to INCREASE in density.
Continue heating the water. What happens?
Eventually, you'll find yourself at an interesting place..... The water and the steam are identical in density. There is no longer a line that delinates water and steam!
So is it a liquid or a gas?
Well, it turns out that it behaves like a gas and so for purposes of conversations such as this one it's probably best just to think of it as a gas. Still, since the material can't simply be compressed to make a liquid the powers that be like to differentiate it from a "normal" gas and call it a "supercritical fluid." But for all intents and purposes... it's a gas.
And the temperature/pressure at which this happens? It's called the "critical point" and can be seen on the pic I included earlier.
Obviously, different materials are going to have different temperatures (and pressures) associated with their critical points. For water, it's somehwere around 700 F. For CO2? It's something like 80 F.
But for nitrogen and oxygen (ie, air)?
I don't remember the numbers off the top of my head but it's DAMNED COLD by human standards. (A moment's google should give you numbers, but I'm not in the mood). The point being that if you compress the air we breath, the best you can do is generate a supercritical fluid. You'll never have liquid air (unless you chilll it).
Posted: Thu Dec 24, 2009 10:33 pm
by Mr.Sandman
If you don't even know what metal larda made his hybrid out of then you have no business attempting this. You honestly sound like you have no idea what you are doing. not to be a jerk but the truth isn't always pleasant. Also please don't double post, it gets really annoying after a while. This is going to be very expensive so your budget should be a minimum of at least $3000. If you are serious about this and actually going to build this and not "disappear", then i would recommend you use some sort of piston design. But like i said i just don't see this happening.
Posted: Thu Dec 24, 2009 10:53 pm
by Ragnarok
D_Hall wrote:But for nitrogen and oxygen (ie, air)?
126.2K and 3.4 MPa for Nitrogen, 154.5K and 5.0 MPa for Oxygen.
In other words, friggin' cold. The coldest recorded (natural) temperature on Earth is -89.2 Centigrade, which works out at 183.9 Kelvin, still 29.4 Kelvin too toasty to compress oxygen down into a liquid.
Posted: Thu Dec 24, 2009 11:00 pm
by velocity3x
10,000 psi? That's 680 atmospheres! How big are you going to make the tank volume? I suggest you start pumping air right now so you'll have enough for one shot by the time the gun is completed and ready to fire!
My guess is that you lack the means to buy or manufacture even modicum of gas compressed to 10,000 psi. If you can, 10,000psi may cause you to depart immediately for the afterlife.
Posted: Thu Dec 24, 2009 11:42 pm
by inonickname
You've already made several gigantic mistakes if you want the most powerful pneumatic cannon. Area increases exponentially by diameter. At twice the area, there will be four times the force on the projectile at the same pressure.
Translation? You could use 10,000 psi in a 1" barrel, then roughly 2500 in a 2" barrel and have the same amount of force exerted. Eventually your 10-thousand psi will only be worth a few hundred as the bore sizes get bigger. Making a large cannon to take these pressures is insanely hard.
Secondly, using air. Unless you design your cannon to specifically heat the working fluid before (and during) the shot you are truly limited by the speed of sound in your working fluid. Muzzle energy increases by the square of the velocity, so as much velocity as possible is desired.
Helium and hydrogen have substantially higher speeds of sound, especially when heated. For optimal performance you would use hydrogen which is heated by a piston or similar means, such as in a light gas gun.
his goes up to 200x mix and the pressure valve is only rated to 5000 psi. if i am wrong in any way please recorrect me. sorry if i sound like a jerk but that's who i am
It might be a good idea to assume that some of us know what we're talking about. The valve is rated for a working pressure of 5000 psi. The spike of 20,000 psi lasts for a minute amount of time due to heat losses and expansion (it is a .2 c:b gun, after all). Lastly, the valve is protected by the equivalent of a needle valve flow route.
it would expand faster and more and as long as their are no leaks it would be not neccasarily as effeicent as a 3000 psi spud gun
In fact, it wouldn't expand faster. You will be limited by the speed of sound in the working fluid. Inefficiencies in your valve will probably prevent it from even reaching SOS in the gas.
I was thinking of using the same metal as "lardas first hybrid
That would be stainless steel, as already suggested. EN 1.4462 high strength stainless to be exact.
There are a few machine/fabrication shops around where I live. I would get an estimate from them.
If your design is of any kind of size and made to any kind of safety factor expect it to run in the thousands.
Perhaps do some reading up on basic physics. It's hard to get around the laws of physics.
Posted: Thu Dec 24, 2009 11:48 pm
by McCoytheLesser
In order to create a chamber that could withstand 10,000 psi, it would have to be made out of very thick (say schedule 120) stainless steel pipe ($$$). ALL joints, flanges, fittings, etc. would have to be TIG welded without any defects in the weld, if there are any defects, everything around the weld has to be scrapped and redone (even higher $$$$).With that in mind, I don't think I'm alone when I say "I don't think it's going to be built." If your just trying make a high pressure gun, try to find an oxygen bottle from a cutting torch. They hold around 2200 psi constantly.
Posted: Thu Dec 24, 2009 11:52 pm
by inonickname
try to find an oxygen bottle from a cutting torch. They hold around 2200 psi constantly.
Make sure you completely purge it and clean it well if you use an oxygen bottle. Oxygen under pressure will be very volatile in the presence of oils, grease etc. A nitrogen tank may be a better option.
