Page 2 of 3
Posted: Tue Oct 10, 2006 7:49 pm
by Anon
Your idea is good. But I would be second guessing not use hydrogen around a electric compressor motor. You need a strong DC power source and for the electrodes stainless steel plates would be the best since often for best results people use salt in the water since salt is a metal makes a better conductor of the water. Also I heard if its in a confined space that it can self-pressurize, is this true?
Posted: Tue Oct 10, 2006 7:55 pm
by cannon freak
It can self pressurize because the volume of gaseous H2 and O2 is a lot more than the volume of water from which it comes. For an electrolyte (Allows current to pass more easily through the water) I would use sodium hydroxide, and as Anon said stainless steel plates for electrodes. Also for a power supply you want high amperage and low voltage.
Posted: Tue Oct 10, 2006 8:04 pm
by sergeantspud2
Alright so I wouldnt be needing a compressor or if you read the bottom... the hose to push out the gases. And stainless steel instead of carbon? Is carbon a close comparison? Im guessing that stainless steel is a lil more expensive than carbon is the way that site suggested it.
Also what would you suggest as a power source?
Posted: Tue Oct 10, 2006 8:16 pm
by cannon freak
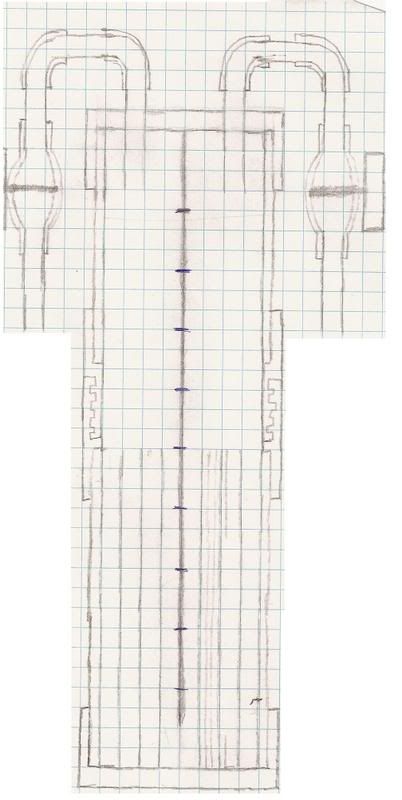
This is what I was planning to build, It would self pressurize. There would be a bunch of stainless steel plates lined up and separated by some kind of wall. Sticking through the wall would be small sections of stainless steel bolts or something just to let current pass more easily, as well there is a small gap at the bottom for the water. It would unscrew from the bottom allowing you to pour water in ( you could attach another small pipe to eliminate this part).
The only problem would be that whatever you are filling with hydrogen you would have to attach another tank 1/2 the size to balance out the pressure. As for a power source, that is the reason I haven't built one because I just can't find a cheap/reliable one.
Sorry for the bad drawing but it was drawn up quickly.
Cannon Freak.
Posted: Tue Oct 10, 2006 10:58 pm
by sergeantspud2
Hey Ive got an idea for the power supply... an old computer power supply. Now tell me if this is going to work... 188 watts and the rest of the info is on <a href="
http://www.wikihow.com/Convert-a-Comput ... pply">this page</a>
Is that going to be enough power?
Posted: Wed Oct 11, 2006 4:59 am
by Anon
Reason for stainless steel is that over time it will last longer than carbon. Hmm using that for a power supply, Im not sure on how big you could make the machine but ya they are DC so it should work.
Posted: Wed Oct 11, 2006 5:10 am
by FreakyShotGlass
An easier way to make hydrogen under pressure is to take a metal chamber and add some HCl (Hydrochloric Acid) and then add Aluminium or Magnesium through a screw on end cap and then screw the end cap back on and wait about 30 secs - 1 minute depending on chamber size and voila.
Posted: Wed Oct 11, 2006 2:09 pm
by zeigs spud
That would probably look like a frickin methlab and i dought my parents would let me do all of that unless i did it outside and they new exactly wht was goin on lol.
But for the practical way of getting all hydrogen into a container is easy. Fill the tube but will all water (till it just barrly overflows) get a highly deflated ballon or mod one of those space storage bags that takes out air and put that ontop (when you take the air outa the storage bag stop when water gets to the hole where your taking air out...der.) and the hydrogen will take up mor volume that what it originated from becuase it has more energy which spaces out the atoms. "easy" -cough- as that..lol
I think it's safe to say that he should go het some comprressed CO2 becuase the "meth lab" idea (which is very inpressing) seems too much work and Helium is too explosive.
EDIT>>>
One question though. i must of read somthing wroung. How do the corbon sticks seperate the hydrogen and oxygen molecules in to the too halfs of the pvc instead of just separating them and then they are free floating gass mixed in the top of the tube? In your picture if you want a closed circut using the water wouldn't the carbon stick need to bee closer, or do you just need opisite polairitys? (s.p.)
Posted: Wed Oct 11, 2006 4:55 pm
by sergeantspud2
Yeah if/when I make it, the stainless steel bars would be closer in order to get more flow. And each of the bars + and - separate different atoms. Like I think the + extracts the hydrogen and the - exracts the oxygen. Im getting all of this from the link I provided im my first post.
LOL yeah I guess it does kind of look like a meth lab!

Your idea about the ballon or spacesaving bags is alright but I want to "compress" the gas. And what the heck does co2 have to do with anything?
And guys whoever looks at the pic next, take out the compressor idea! I droped that because firstly, a small spark could ignite an explosion and secondly, the gas already compresses after it separates.
And last thing... The computer power source will that provide enough amps and volts? Is it volts or amps im looking for?
Posted: Wed Oct 11, 2006 5:16 pm
by cannon freak
The hydrogen comes off of the - electrode and the oxygen off of the + electrode, you don't want to get them mixed up lol.
what are you planning on using for an electrolyte, don't use salt, you will get pure H2 but also C2 and the Na that comes off of the positive electrode will turn the water into a more and more basic solution. I would suggest sodium hydroxide.
As for a power supply it all depends on the surface area of the electrodes and the conductivity of the water, the more surface area and more conductivity the more amps you would draw. The computer power source may provide enough although it probably will be slow because of the limited amperage.
Cannon Freak
Posted: Wed Oct 11, 2006 5:22 pm
by sergeantspud2
Alight thanks for the + - correction. Where would I get sodium hydroxide? Is it going to produce any other products other than oxygen and hydrogen? And what is a good amperage to get sufficent results?
Posted: Wed Oct 11, 2006 5:26 pm
by cannon freak
For sodium hydroxide you can get it in the form of Lye I believe in any large stores, its used for cleaning I think.
I'm really not the one to ask about the amperage stuff because I don't fully get how it works either. Ive seen some that have drawn like 50 - 60 amps or so, but I think power supply would work, it might just be slow.
I'm pretty sure that that is how it works.
Cannon Freak.
Posted: Thu Oct 12, 2006 1:21 am
by L.J.R
An easier way to make hydrogen under pressure is to take a metal chamber and add some HCl (Hydrochloric Acid) and then add Aluminium or Magnesium through a screw on end cap and then screw the end cap back on and wait about 30 secs - 1 minute depending on chamber size and voila.
I don't think there is enough control in that, I thought of something similair but having the hydrogen run through cold water to condense the water from all the heat made during the reaction. If you ran it through iced water into a steel chamber with a pressure gauge and 1/4 ball valve you could wait for the gass to warm up and make more pressure and if you had to much pressure let some out throught the ball valve.
I was going to use a computer PSU (power supply unit) to make an electrolytic cell to convert NaCl "salt" to NaClo3"sodium chlorate" and Kcl to KClo3.
Posted: Thu Oct 12, 2006 1:26 am
by L.J.R
An easier way to make hydrogen under pressure is to take a metal chamber and add some HCl (Hydrochloric Acid) and then add Aluminium or Magnesium through a screw on end cap and then screw the end cap back on and wait about 30 secs - 1 minute depending on chamber size and voila.
I don't think there is enough control in that, I thought of something similair but having the hydrogen run through cold water to condense the water from all the heat made during the reaction. If you ran it through iced water into a steel chamber with a pressure gauge and 1/4 ball valve you could wait for the gass to warm up and make more pressure and if you had to much pressure let some out throught the ball valve.
I was going to use a computer PSU (power supply unit) to make an electrolytic cell to convert NaCl "salt" to NaClo3"sodium chlorate" and Kcl to KClo3, but thats neither here or there. But I think an ATX PSU would be sufficient to make hydrogen unless your impatient.
And if you want sodium hydroxide "NaOH" look for caustic soda or drain opener only get the stuff that is white crystals nothing with colours.
I think I might just look for helium instead of going through the trouble to get hydrogen.
Posted: Thu Oct 12, 2006 6:07 pm
by zeigs spud
I'm not sure if i am getting this correctly. so does there have to be a complete circut for this prosses to work? if so then the tubes (vertical ones) will need to be quite close together. as for a conducter i say that copper is the best conducter for this. Power source wise mabey a car battery if you wana push it lol?
I have the polaritys wroung on this so ignore that but this is how i would do it if i were you.
<a href="
http://img138.imageshack.us/my.php?imag ... gennx6.png" target="_blank"><img src="
http://img138.imageshack.us/img138/7117 ... nx6.th.png" border="0" alt=""></a>
what you would do is put an endcap (screw on) onto the right side and a permenet one onto the left side. then once it is built, flip it upside down take off the cap on the bottom and add water till it overflows (or to get rid of all the air just fill in the bathtub and put the cap back on under the water). then flip it up right and take the right cap off seeing as you want be collecting oxygen and it will make the prsses easyer as it wont be compressing from the build of of the hydrogen taking up more volume than the water and pushing it out on the right side. Once the right cap is off just connect the power source to the conducters and wait (idk how long) once the majority of the water has spilled out and you can see that just barrly tipping the structure to the left makes bubbles come up you can then turn off the power source and put a cap onto the right side (with a scharder valve put into it.). make sure it fits tightly. then add air till the psi is almost at the pvc pipes limit or if its not yet there till the air that you can see on the right tube (through the clear pvc pip) is almost reachign the bottom the the clear section.
from there you have your compressed hydrogen

i just don't knwo how your gonna get it out tho...i'll work on that if what i posted is correct.
