Difference between revisions of "Butane"
| (5 intermediate revisions by 5 users not shown) | |||
| Line 1: | Line 1: | ||
| − | [[Image:Butane.gif|frame|The butane molecule]]Butane is quite | + | [[Image:Butane.gif|frame|The n-butane molecule]]Butane is quite similar to [[propane]], but with a slightly lower [[stoichiometric]] value of 3.23%. Pure butane is found in disposable lighters and lighter refill canisters. |
| − | Butane has a lower vapor pressure than propane, and its boiling point is -0.5°C (31.1°F), | + | Butane has a lower vapor [[pressure]] than propane, and its boiling point is -0.5°C (31.1°F), limiting it's use to higher temperature environments. It is most easily used the same way as [[aerosol]] [[fuel]]s. |
| − | Disposable canisters with mixtures of butane, propane and (sometimes) isobutane | + | Disposable canisters with mixtures of butane, propane and (sometimes) isobutane are sold for camping gear and blowtorches, this gas evaporates at lower temperatures and can be used in cold weather. |
| + | Molecular Formula: C<sub>4</sub>H<sub>10</sub><br> | ||
| + | n-Butane Extended Formula: CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>3</sub><br> | ||
| + | iso-Butane Extended Formula: CH<sub>3</sub>CH(CH<sub>3</sub>)CH<sub>3</sub><br> | ||
| + | Combustion: 2C<sub>4</sub>H<sub>10</sub> + 13O<sub>2</sub> ==> 8CO<sub>2</sub> + 10H<sub>2</sub>O | ||
| + | |||
| + | |||
| + | [[file:Butane_pressure_graph.gif|frame ]] | ||
[[category:fuels]] | [[category:fuels]] | ||
Latest revision as of 00:33, 21 February 2011
Butane is quite similar to propane, but with a slightly lower stoichiometric value of 3.23%. Pure butane is found in disposable lighters and lighter refill canisters.
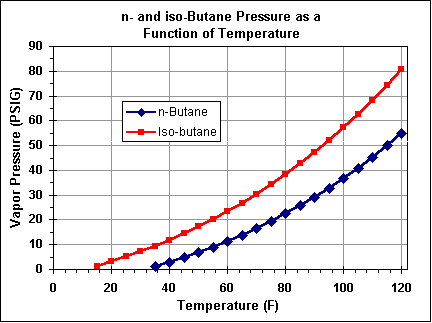
Butane has a lower vapor pressure than propane, and its boiling point is -0.5°C (31.1°F), limiting it's use to higher temperature environments. It is most easily used the same way as aerosol fuels.
Disposable canisters with mixtures of butane, propane and (sometimes) isobutane are sold for camping gear and blowtorches, this gas evaporates at lower temperatures and can be used in cold weather.
Molecular Formula: C4H10
n-Butane Extended Formula: CH3CH2CH2CH3
iso-Butane Extended Formula: CH3CH(CH3)CH3
Combustion: 2C4H10 + 13O2 ==> 8CO2 + 10H2O